# Unraveling the Mysteries of Water: 5 Questions We Still Have
Written on
Chapter 1: The Enigma of Water
What complexities lie beneath the surface of something as seemingly simple as water? It’s often described as wet, clear, and essential for life. Yet, many people, like my late mother who lived to 99, found it mundane, questioning why taxpayer funds are allocated for water research. However, a contrasting group, influenced by pseudoscience, promotes fantastical theories about water's properties. The reality is that while water is abundant—ranking as the third most prevalent molecule in the universe—it is also far more intricate than one might assume.
1. How Many Types of Ice Exist?
As of now, researchers have identified 17 distinct crystalline forms of ice, with Ice I? being the most common on Earth. Another form, Ice Ic, appears in minimal quantities in the upper atmosphere, while the remaining 15 forms require extreme pressure to exist. Interestingly, even in the vastness of interstellar space, water takes on a non-crystalline, glass-like state. The diversity in ice forms stems from the tetrahedral arrangement of strong hydrogen bonds between water molecules. In Ice I?, for instance, these bonds create an open, low-density structure.
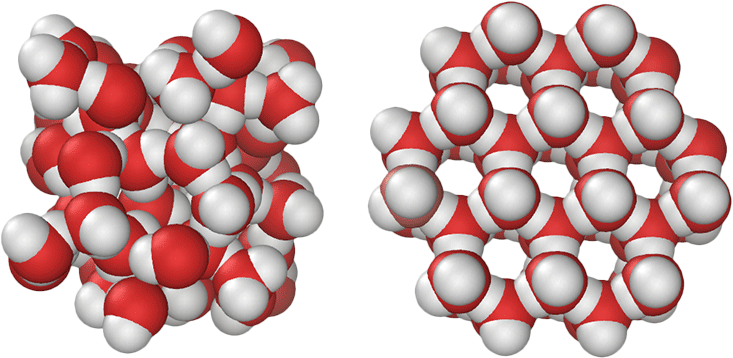
The application of pressure to substances like ice can lead to the transformation of low-density structures into denser forms, potentially revealing even more ice types yet to be discovered.
2. Are There Two Variants of Liquid Water?
Years ago, Japanese researchers suggested that high-pressure conditions might cause liquid water to exist in two different phases, akin to its amorphous ice state. This theory posits a normal, low-density water and a compact, high-density variant. Simulations have supported this idea, showing phase transitions in supercooled water. However, some scientists argue these findings could be artifacts, complicating our understanding of water’s behavior at extreme conditions.
3. What is the Mechanism of Water Evaporation?
The evaporation rate of water remains a significant uncertainty in climate modeling, influencing cloud properties and their interaction with light. Despite its importance, the exact mechanics of evaporation are not fully understood. Traditionally, it has been modeled through molecule collision rates and a variable coefficient. Recent research indicates that the coefficient may be close to one, while other studies have produced lower values under realistic atmospheric conditions. Additionally, factors like surface tension and salt content complicate our understanding further.
We Still Don't Understand What Water Is, Here's Why
In this video, scientists delve into the complexities of water and its intriguing properties, shedding light on why it remains an area of active research.
4. Is the Surface of Liquid Water Acidic or Basic?
The mist surrounding waterfalls, like Niagara Falls, presents a puzzling phenomenon where water droplets appear to carry a negative charge, leading to the assumption of a basic surface pH. Contrary to this belief, recent studies indicate that proton exchanges at the water's surface may render it acidic, which has significant implications for various chemical and biological processes.

5. Does Nanoconfined Water Behave Differently?
Water does not merely exist in vast bodies; it often finds itself in confined spaces, such as carbon nanotubes and xerogels. Research suggests that when water is trapped in these tiny regions, it may exhibit quantum mechanical behaviors distinct from bulk water, potentially impacting everything from biological systems to technological applications. However, further investigation is needed to fully understand the properties of confined water.

Chapter 2: The Science of Water
What Biology Class Didn't Want You To Know About Water
This episode of Marvels of the Science explores the hidden intricacies of water that biology classes often overlook, revealing critical insights into its behavior and properties.
Richard Saykally, a chemistry professor at the University of California, Berkeley, has made significant contributions to our understanding of water, with over 400 publications to his name. Despite its commonality, water continues to present remarkable mysteries that scientists strive to unravel.